Of the following transitions in the Bohr hydrogen atom, which transition results in the emission of photons
Question:
Of the following transitions in the Bohr hydrogen atom, which transition results in the emission of photons with the shortest wavelength?
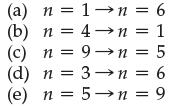
Transcribed Image Text:
n = 6 (a) (b) (c) n=1 n =4 n = 1 = 5 n=9→n (d) n = 3→n = 6 (e) n = 5→n = 9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
The transition that results in the emission of photons with the shortest wavelength in the Bohr hydr...View the full answer

Answered By

Krishnavendra Y
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagiarism), well-researched and critically analyzed papers.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Consider only transitions involving the n = 1 through n = 4 energy levels for the hydrogen atom (see Figures 6.7 and 6.10). (a) How many emission lines are possible, considering only the four quantum...
-
The cosmic dawn that preceded the epoch of reionization can be probed by low frequency CMB observations using a special radio hyperfine line emitted and absorbed by hydrogen atoms. This line is...
-
Consider only transitions involving the n = 1 through n = 5 energy levels for the H atom (see Figures 6.7 and 6.10). (a) How many emission lines are possible, considering only the five quantum...
-
Name: PN 200 Fundamentals of Nursing II Medication Error Prevention-OTC's Date: Jeff Voss, a 29-year-old graduate student is at the student health center for a physical examination required before...
-
What are six principles of good budgeting?
-
The Adjusted Trial Balance section of the worksheet for Harmon Farm Supply follows. The owner made no additional investments during the year. Prepare a post-closing trial balance for the firm on...
-
What is the difference in accounting for a cash flow hedge and a fair value hedge? LO9
-
The fiscal year for Hokkaido Company ends on May 31. Results for the year ended May 31, 20X1, included the following (in millions of Japanese yen except for number of shares outstanding): Cash and...
-
Brianna has just started her degree at Carleton and because she is a superstar and has the time management skills, she will be working part-time while studying. She now only has to decide between 2...
-
When a hydrogen atom is excited in a flame, a line of blue-violet light is emitted. This happens when an electron makes a transition between the n = 5 and the n = 2 orbit of the atom. If the energy...
-
In which group and period would you expect to find the MOST nonmetallic element?
-
During 20x2, Union Chemical Company had cost of goods sold of \(3,800,000\). The ending balance of LO 4 Cash Payments for Purchases Inventory was 510,000 in \(20 \times 1\) and 420,000 in \(20 \times...
-
Question 37 Plantito Inc., produces potted plants. For next year, Pietro predicts that 45,000 units will be produced, with the following total costs: Direct materials Direct labor ? 80,000 Variable...
-
When you are to design a data transmission system, you have two key considerations to work with: data rate and distance, with emphasis placed on achieving the highest data rates over the longest...
-
How much work does a supermarket checkout attendant do on a can of soup he pushes 0.600 m horizontally with a force of 5.00 N? Express your answer in joules and kilocalories. 3 . (a) Calculate the...
-
Suppose in its income statement for the year ended June 30, 2022, The Clorox Company reported the following condensed data (dollars in millions). Salaries and wages expenses$460 Research and...
-
Consider the extensive form game show in the figure below. How many strategies does Player 2 have in this game? (2,2,1) b (2,4,2) 03 by 03 02 dz (4.2,0) (2.0.2) (0.3.4) (3,5,3) (3,1,2)
-
If r(t) = sin 2t i + cosh t j and h(t) = ln (3t - 2), find Dt[h(t)r(t)].
-
Which should drive action planning more, strengths or weaknesses? That is, is it more important to build on your strengths or to reduce your weaknesses? Explain.
-
Why isnt the motion of a human being described by the Schrdinger equation rather than Newtons second law if every atom in our body is described by quantum mechanics?
-
Explain the following statement: If h = 0, it would be possible to measure the position and momentum of a particle exactly and simultaneously.
-
Why is (p 2 ) rather than p used to calculate the relative uncertainty for the particle in the box?
-
Your company produces a health magazine. Its sales data for 1 - year subscriptions are as follows: Year of Operation Subscriptions Sold % Expired at Year End 2 0 2 0 $ 3 0 0 , 0 0 0 5 0 2 0 2 1 $ 6 4...
-
The adjusted trial balance for Tybalt Construction on December 3 1 of the current year follows. TYBALT CONSTRUCTION Adjusted Trial Balance December 3 1 Number Account Title Debit Credit 1 0 1 Cash $...
-
( US$ millions ) 1 2 / 3 1 / 2 0 1 4 1 2 / 3 1 / 2 0 1 3 1 2 / 3 1 / 2 0 1 2 1 2 / 3 1 / 2 0 1 1 Net income $ 1 4 , 4 3 1 $ 1 2 , 8 5 5 $ 1 0 , 7 7 3 $ 9 , 7 7 2 Depreciation 3 , 5 4 4 2 , 7 0 9 1 ,...

Study smarter with the SolutionInn App


