The trend is for first ionization energy to increase from left to right across a period. Describe
Question:
The trend is for first ionization energy to increase from left to right across a period. Describe any exceptions to this overall trend. Use the portion of the plot that is for the third period atoms to answer this question.
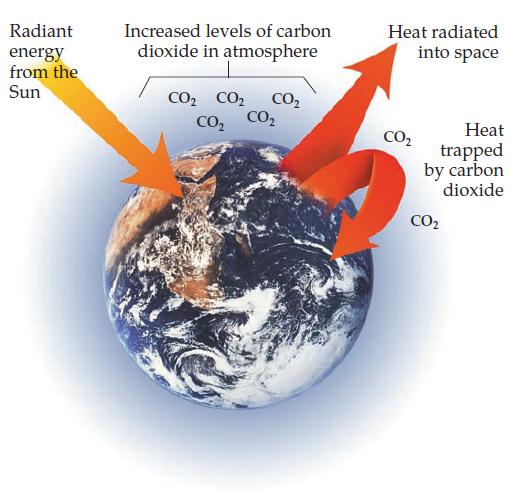
Transcribed Image Text:
Radiant energy from the Sun Increased levels of carbon dioxide in atmosphere CO₂ CO₂ CO₂ CO₂ CO₂ Heat radiated into space CO₂ Heat trapped by carbon dioxide CO₂
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
The accompanying graphs show the first ionization energies and electron affinities of the period 3 elements. Refer to the graphs to answer the questions that follow. a. Describe the general trend in...
-
The Crazy Eddie fraud may appear smaller and gentler than the massive billion-dollar frauds exposed in recent times, such as Bernie Madoffs Ponzi scheme, frauds in the subprime mortgage market, the...
-
Neer Department Store uses the retail inventory method to estimate its monthly ending inventories. The following information is available for two of its departments at August 31, 2011. Sporting Goods...
-
Impact of Transactions Involving Contingent Liabilities on Statement of Cash Flows From the following list, identify whether the change in the account balance during the year would be reported as an...
-
Kalyagin Investments acquired $220,000 of Jerris Corp., 7% bonds at their face amount on October 1, 2016. The bonds pay interest on October 1 and April 1. On April 1, 2017, Kalyagin sold $80,000 of...
-
Explain the difference between a product mixs breadth and a product lines depth.
-
Alberton Co. uses a standard costing system to account for its production of toys. Plastic is added at the start of production; labor and overhead are incurred at equal rates throughout the process....
-
Kennedy, Inc., reported the following data: Net income $199,016 Depreciation expense 14,737 Loss on disposal of equipment (10,696) Gain on sale of building 19,310 Increase in accounts receivable...
-
Give the full symbol for the +2 alkaline earth cation that is in the fourth period and has a mass number 21 greater than its atomic number. Explain.
-
What is the trend in first ionization energy as you go down a given group in the periodic table? As you go across a period from left to right?
-
Why was the Bretton Woods system of pegged yet adjustable exchange rates reasonably successful until 1958 and increasingly unstable thereafter?
-
Part 1 of 4 05 points abook Print References Required information Problem 24-2A (Algo) Payback period, accounting rate of return, net present value, and net cash flow calculation LO P1, P2, P3 [The...
-
Keenan Music's CEO has been pondering about the recent proposal of the Specialty Guitar Project. The accountant has done a capital budgeting analysis on the project and outlined the conditions that...
-
On July 1, 2025, Sheridan Co. pays $15,000 to Blue Spruce Insurance Co. for a 2-year insurance contract. Both companies have fiscal years ending December 31. (a1) Journalize the entry on July 1 and...
-
A CU triaxial test with c = 20 psi is performed on a sand and a deviator stress of 80 psi fails the specimen. Previous tests revealed that the effective friction angle for this sand is 35. Calculate...
-
Haliburton Mills Inc. is a large producer of men's and women's clothing. The company uses standard costs for all of its products. The standard costs and actual costs for a recent period are given...
-
Consider the data set of 30 piston rod lengths given in DS 6.2.3. Calculate the sample mean, sample median, sample trimmed mean, and sample standard deviation. Calculate the upper and lower sample...
-
Carlton Stokes owns and operates a car-detailing business named SuperShine & Detailing. For $150, Carltons business will hand wash and wax customers cars, vacuum the interior, and thoroughly clean...
-
A rigid vessel of volume 0.400 m 3 containing H 2 at 21.25C and a pressure of 715 10 3 Pa is connected to a second rigid vessel of volume 0.750 m 3 containing Ar at 30.15C at a pressure of 203 10 3...
-
A mixture of oxygen and hydrogen is analyzed by passing it over hot copper oxide and through a drying tube. Hydrogen reduces the CuO according to the reaction CuO(s) + H 2 (g) Cu(s) + H 2 O(l), and...
-
Many processes such as the fabrication of integrated circuits are carried out in a vacuum chamber to avoid reaction of the material with oxygen in the atmosphere. It is difficult to routinely lower...
-
1. Determine the value of the right to use asset and lease liability at commencement of the lease.
-
Problem 22-1 The management of Sunland Instrument Company had concluded, with the concurrence of its independent auditors, that results of operations would be more fairly presented if Sunland changed...
-
Question 4. - Week 9. What are the major competitive issues General Electric faces when managing cooperative strategies? - (7 marks)

Study smarter with the SolutionInn App


