Working backward, deduce the starting material that led to the indicated product through the defined reactions. (a)
Question:
Working backward, deduce the starting material that led to the indicated product through the defined reactions.
(a)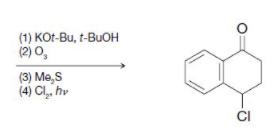
(b)
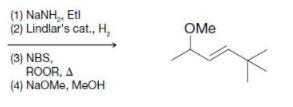
Transcribed Image Text:
(1) KOt-Bu, t-BuOH (2) 0₂ (3) Me,S (4) Cl₂.hv CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
a The starting material is 2chloropropane b The starting material is 23dichlorobutane E...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry
ISBN: 978-1118875766
12th Edition
Authors: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Question Posted:
Students also viewed these Sciences questions
-
Working backwards, deduce the starting material that led to the indicated product through the defined reactions. (a) (b) (c) Br (1) NaNH,, Mel (2) Br, (1 equiv) A - Br
-
Draw the structure of the starting material that would undergo azir-elimination give the -E isomer of the alkene product in the E2 reaction of Eq. 9.40.
-
Working backward from data that has eliminated intercompany transactions. (Adapted from a problem by S. A. Zeff.) Alpha owns 100% of Omega and consolidates Omega in an entity called Alpha/Omega....
-
1. Which type contains a single character enclosed within single quotes? A. Character B. Numeric C. Floating point 2. The modulus operator uses, B. - B. < A. + 3. Every variable should be separated...
-
3b + 2 > 7b 6 Describe the solution set as an inequality, in interval notation, and on a graph.
-
Real estate prices depend, in part, on property size. The house size X (in hundreds of square feet) and house price Y (in thousands of dollars) of a random sample of houses in a certain county were...
-
34. Jayhawk Company reports current E&P of $300,000 and a deficit in accumulated E&P of ($200,000). Jayhawk distributed $400,000 to its sole shareholder, Christine Rock, on the last day of the year....
-
Revenue recognition at and after time of sale. Assume that during December 2008, Nordstrom sold $20 million of merchandise and another $12 million of gift cards, of which $24 million was on credit...
-
What are 3 major peaks and valleys that Tesla Inc has gone through in the stock market? What business, political, or economic reasons caused these peaks and valleys?
-
Two students are preparing for their micro exam, but they seem confused: Student A: "We learned that demand curves always slope downward. In the case of a competitive firm, this downward-sloping...
-
For each of the following, identify the product (represented by A and B) that would be formed through the indicated sequence of steps from the given starting material.
-
For each of the following questions, please provide a route that could reasonably be expected to convert the starting material into the final product. In each case, more than one reaction is...
-
Use your answer to Problem 27 to determine the relative pK values of N3 in cytosine and in uracil. Data from Problem 27: The pK value for N1 of adenine is 3.64, whereas the pK value for N1 of guanine...
-
The adjusted trial balance columns of a worksheet for Levitt Corporation are shown below. The worksheet is prepared for the year ended December 31, Complete the worksheet by (a) entering the adjusted...
-
Derive the commutator $\left[Q_{i}, Q_{j} ight]=i \epsilon_{i j k} Q_{k}$ for the charge defined in Eq. (33.4). Use the charge (33.4) to write the commutator, displaying explicit matrix indices...
-
Verify that the potential $V(\pi, \sigma)$ can be written as Eq. (33.11), and that if $\epsilon=0$ and the symmetry is implemented in the Wigner mode the masses for the $\pi$ and $\sigma$ fields are...
-
Figure 5.7 shows a number of yield curves at various points in time. Go to www.treasury.gov, and in the Resource Center at the top of the page click on Data and Charts Center. Find the Treasury yield...
-
The number of vacation days used by a sample of 20 employees in a recent year In Exercises 2326, use technology to draw a box-and-whisker plot that represents the data set. 3 9 2 17 5 3 2 2 6 4 0 10...
-
How did the National Securities Markets Improvement Act of 1996 (NSMIA) change the regulatory structure of the securities industry?
-
If a test has high reliability. O the test measures what the authors of the test claim it measures O people who take the same test twice get approximately the same scores both times O scores on the...
-
Show both the substitution and elimination products that are formed in these reactions: a) C CI + CH0 CHOH + OH HO EtOH b) Br + CHOH CH3OH
-
Show the rearranged carbocations that are expected from these carbocations: a) +CH CH3 b) CHCHCHCHCH c) CH3
-
Show the substitution products for these reactions: a) b) +CHCHOH Br CI Ja + HO EtOH HO EtOH
-
Apple inc cash flow
-
Assume todays settlement price on a CME EUR futures contract is $1.3142 per euro. You have a short position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Determining ending consolidated balances in the second year following the acquisition-Equity method Assume that your company acquired a subsidiary on January 1, 2012. The purchase price was $650,000...

Study smarter with the SolutionInn App


