Give the structure of the organic product expected when CH 2 I 2 reacts with each of
Question:
Give the structure of the organic product expected when CH2I2 reacts with each of the following alkenes in the presence of a Zn–Cu couple: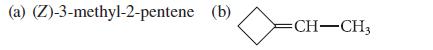
Transcribed Image Text:
(a) (Z)-3-methyl-2-pentene (b) CH-CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
a b HC H CHCH3 CH3 ...View the full answer

Answered By

Anjali Arora
Having the experience of 16 years in providing the best solutions with a proven track record of technical contribution and appreciated for leadership in enhancing team productivity, deliverable quality, and customer satisfaction. Expertise in providing the solution in Computer Science, Management, Accounting, English, Statistics, and Maths.
Also, do website designing and Programming.
Having 7 yrs of Project Management experience.
100% satisfactory answers.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the structure of the expected product formed when benzylamine reacts with each of the following reagents: (a) Hydrogen bromide (b) Sulfuric acid (c) Acetic acid (d) Acetyl chloride (e) Acetic...
-
Give the structure of the expected product formed when benzylamine reacts with each of the following reagents: (a) Hydrogen bromide (b) Sulfuric acid (c) Acetic acid (d) Acetyl chloride (e) Acetic...
-
Give the structure of the product formed when each of the following alkenes reacts with bromine in water: (a) 2-Methyl-1-butene (c) 3-Methyl-1-butene (b) 2-Methyl-2-butene (d) 1-Methylcyclopentene
-
The Acme Insurance Company purchased a five-year bond whose interest rate floats with LIBOR. Specifically, the interest rate in a given year is equal to LIBOR plus 200 basis points. At the same time...
-
Assume at the beginning of 2012, the Ashlawn Village Street and Highway Fund (a special revenue fund) has cash of $300,000 offset by assigned fund balance in the same amount. 1. During the year, the...
-
The consolidated balance sheet of Pan Corporation and its 80 percent subsidiary, Sun Corporation, contains the following items on December 31, 2014 (in thousands): Cash...
-
2. Dar invests $75,000 cash in the partnership for a 25 percent interest in the partnership capital and profits, and partnership assets are revalued.
-
Starbucks is opening new stores abroad every day, it seems. If you were in charge, would you use expatriate managers or host-country nationals to staff the new facilities? Explain your thinking.
-
Cash $200,000 Marketable Securities $10,000 Accounts Receivable $300,000 Inventories $10,000 Prepaid Expenses $10,000 Land $100,000 Building $1,000,000 China, glass, etc. $200,000 Accumulated...
-
Predict the products that result when each of the following alkenes reacts with chloroform and potassium tert-butoxide. Give the structures of all product stereoisomers, and, if more than one...
-
(a) Give the structures of two isomeric alkylmagnesium bromides that would react with water to give propane. (b) What compounds would be formed from the reactions of the reagents in (a) with D 2 O?
-
Why must criminal statutes carefully and clearly define the prohibited behavior?
-
Write out the form of the partial fraction decomposition of the function (See Example ). Do not determine the numerical values of the coefficients. (If the partial fraction decomposition does not...
-
Below is the actual assignment information. Here is where you will submit your event for approval. It is not graded, but you will need it to be marked complete in order to submit your paper, so...
-
3. (20 points) A researcher is interested in whether the phonics method of teaching reading is more or less effective than the sight method, depending on what grade the child is in. Twenty children...
-
Let A and B be the matrices given below: -5 9 -7 A= 8 -1 -3 B=9 6 -1 8 -1 -7. 0 Perform the following matrix operations and enter the entries below: -4A = A-4B = 5A-3B=
-
The product business can be isolated into four principal classes: programming administrations, framework administrations, open source and SaaS. The accompanying depicts the classifications of...
-
Find the sum of the series? 2k (2k+1-1)(2t-1)
-
In what ways does a well-designed enterprise search software vary from popular search engines (e.g., Bing, DuckDuckGo, and Google)?
-
Hydrocarbon A has the formula C 9 H 12 and absorbs 3 equivalents of H 2 to yield R, C 9 H 18 , when hydrogenated over a Pd/C catalyst. On treatment of A with aqueous H 2 SO 4 in the presence of...
-
How would you carry out the followingreactions? (a) CCH-CCH3 CH3CH2C=CH () H2C%3CH CH3CH2CH2CHO .3- (c) 3C (d) CH . CH 7, 22H (e) CH3CH2C=CH (f) CH3CH2CH2CH2CH=CH2 CHCH2CH2CH2C%CH (2 steps)
-
Occasionally, chemists need to invert the stereochemistry of an alkene?that is, to convert a cis alkene to a trans alkene, or vice versa. There is no one-step method for doing an alkene inversion,...
-
Chapter o Homew ebook 50,000-unit production quantity: $ 227,049 7 70,000-unit production quantity: $ 66,751 d. In addition to mean profit, what other factors should FTC consider in determining a...
-
Diamond makes downhill ski equipment. Assume that comic has offered to produce ski poles for Diamond for $20 per pair Diamond needs 200,000 pairs of poles per period Diamond can only avoid 5150,000...
-
17? Which of the following statement is true Select one: a. All evidence must have the same level of reliability b. All evidence must have the same level of persuasiveness C. All are false d....

Study smarter with the SolutionInn App


