In each of the following cases, the labeled protons are constitutionally equivalent. Determine whether the labeled protons
Question:
In each of the following cases, the labeled protons are constitutionally equivalent. Determine whether the labeled protons in each case are expected to have identical or different chemical shifts.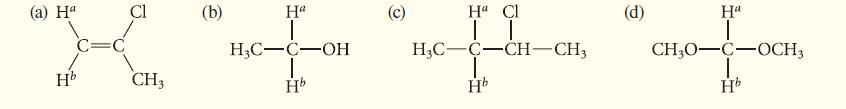
Transcribed Image Text:
(a) Ha Hb Cl CH3 (b) Ha 1 H3C-C-OH Hb O Ha Cl | | H3C-C-CH-CH3 Hb (d) Ha 1 CH3O-C-OCH3 Hb
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Apply the principles of Sec 109A to determine whether the protons in question are chemically equivalent If the two protons are chemically equivalent t...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Based on Westlaw's data on the case Christoff v. Nestl USA, INC., I did the brief case. My question is, is there any need to add and adjust the content and form of the case brief, especially the...
-
Specify whether the labeled protons in each of the following structures would be expected to have the same or different chemical shifts. (a) (b) CH3 H,C CH3 HC C-CH CI Hb
-
You are given the following as it relates to the price of a pound of flour in Adam Island, 2016 to 2022: 2016-$1.35 2017 - $1.50 2018 - $1.65 2019 - $1.85 2020 - $2.15 2021 - $2.00 2022 - $2.10 Using...
-
What effect does a debit memo in a bank statement have on the Cash account? What effect does a credit memo in a bank statement have on the Cash account?
-
A system consists of five identical components connected in series as shown: As soon as one component fails, the entire system will fail. Suppose each component has a lifetime that is exponentially...
-
What are the risks associated with such rapid and dispersed growth for a new company? L01
-
What if you conducted a PAQ analysis that indicated that the Wonderlic was a valid test for this job? Do you believe that this result establishes the legality of the Wonderlic? Given that the...
-
the company Goldman Sachs Discuss the following: Performance section should use profitability (profit margin, return on assets and return on equity) and investment ratios (earnings per share,...
-
Determine the structure of the compound with the formula C 6 H 13 Cl that has the NMR spectrum shown in Fig. 13.13. FIGURE 13.13 The NMR spectrum for Study Problem 13.4. 2400 absorption 8 2100 7 1800...
-
A compound contains carbon, hydrogen, oxygen, and one nitrogen. Classify each of the following fragment ions derived from this compound as an odd-electron or an even-electron ion. Explain. (a) The...
-
The adoption of a certain brand of shoe or apparel by athletes can be a powerful influence on students and other fans. Should secondary school and university coaches be paid to determine what brand...
-
Molina Company produces three products: A130, B324, and C587 All three products use the same direct material, Brac Unt data for the three products are in the provided table. (Click to view the unit...
-
MFGE 437 S21 - Homework 1 Submissions will be Online! Please scan your HWs and upload on Canvas Problem 1: A vertical milling machine is to be retrofitted with three identical DC servo motors. The...
-
On January 1, Palisades, Inc., acquired 100 percent of Sherwood Company's common stock for a fair value of $120,340,000 in cash and stock. The carrying amounts of Sherwood's assets and liabilities...
-
(1) A test balloon has an accelerometer attached to it. After you release it and start collecting data it is 5 ft in front of you and 16 ft above you, and it is moving 5 ft/s to your left and 4 ft/s...
-
484 ... Age of Accounts as of June 30, 2019 1-30 31-60 61-90 Over 90 Customer Name Days Days Days Days Total Balance Canyon Youth Club $ 250 $ 250 Crazy Tees 200 $ 150 350 Early Start Daycare $500...
-
In Figure 5, let D be the area of triangle ABP and E the area of the shaded region. (a) Guess the value of By looking at the figure. (b) Find a formula for D/E in terms of t. (c) Use a calculator to...
-
State whether each of the following will increase or decrease the power of a one-way between-subjects ANOVA. (a) The effect size increases. (b) Mean square error decreases. (c) Mean square between...
-
There are four resonance structures for anthracene, one of which is shown. Draw the otherthree. Anthracene
-
There are five resonance structures of Phenanthrene, one of which is shown. Draw the otherfour. Phenanthrene
-
Look at the five resonance structures for Phenanthrene (Problem 15.26) and predict which of its carboncarbon bonds is shortest. Phenanthrene
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


