Determine the structure of the compound with the formula C 6 H 13 Cl that has the
Question:
Determine the structure of the compound with the formula C6H13Cl that has the NMR spectrum shown in Fig. 13.13.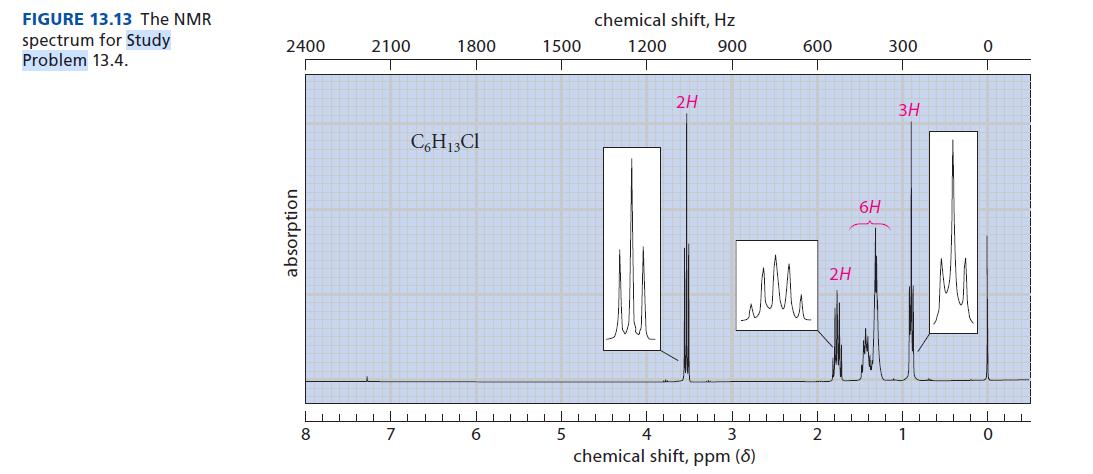
Transcribed Image Text:
FIGURE 13.13 The NMR spectrum for Study Problem 13.4. 2400 absorption 8 2100 7 1800 C6H13Cl 6 1500 chemical shift, Hz 1200 900 TIL 5 2H 600 Me 4 3 chemical shift, ppm (8) 2 2H 6H 300 3H 1 0 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The unknown compound has an unsaturation number of zero and is therefore an alkyl chloride The spect...View the full answer

Answered By

Salmon ouma
I am a graduate of Maseno University, I graduated with a second class honors upper division in Business administration. I have assisted many students with their academic work during my years of tutoring. That has helped me build my experience as an academic writer. I am happy to tell you that many students have benefited from my work as a writer since my work is perfect, precise, and always submitted in due time. I am able to work under very minimal or no supervision at all and be able to beat deadlines.
I have high knowledge of essay writing skills. I am also well conversant with formatting styles such as Harvard, APA, MLA, and Chicago. All that combined with my knowledge in methods of data analysis such as regression analysis, hypothesis analysis, inductive approach, and deductive approach have enabled me to assist several college and university students across the world with their academic work such as essays, thesis writing, term paper, research project, and dissertation. I have managed to help students get their work done in good time due to my dedication to writing.
5.00+
4+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the structure of a compound with molecular formula C 5 H 10 O that exhibits the following broadband-decoupled and DEPT-135 spectra. The DEPT-90 spectrum has no signals. Broadband-decoupled...
-
Deduce the structure of a compound with molecular formula C 9 H 10 O 2 that produces the following 1 H NMR spectrum and 13 C NMR spectrum: Proton NMR 10 Chemical Shift (ppm) Carbon NMR - 128.4 128.8-...
-
Deduce the structure of a compound with molecular formula C 5 H 10 O that exhibits the following IR, 1 H NMR, and 13 C NMR spectra. Data from the mass spectrum are also provided. 100 Mass Spec. Data...
-
The stockholders' equity accounts of Whispering Company have the following balances on December 31, 2025. Common stock, $10 par, 304,000 shares issued and outstanding $3,040,000 Paid-in capital in...
-
What information is normally included in a bank statement?
-
The two-parameter gamma distribution can be generalized by introducing a third parameter , called a threshold or location parameter: replace x in (4.8) by x - and x 0 by x . This amounts to...
-
Beyond simply increasing revenue, what advantages might a new business benefit from thanks to early international exposure and growth? L01
-
Louisville Corporation produces baseball bats for kids that it sells for $32 each. At capacity, the company can produce 50,000 bats a year. The costs of producing and selling 50,000 bats are as...
-
Se le proporciona la siguiente informacin para Troiano Pizza Company: Ventas = $80 000; Costos = $33,900; Adicin a ganancias retenidas = $7,300; Dividendos pagados = $2,460; Gastos por intereses =...
-
A compound C 8 H 18 O 2 with a strong, broad infrared absorption at 3293 cm 1 has the following proton NMR spectrum: (The resonance at d 1.96 disappears when the sample is shaken with D 2 O.) The...
-
In each of the following cases, the labeled protons are constitutionally equivalent. Determine whether the labeled protons in each case are expected to have identical or different chemical shifts....
-
Will you use psychological tests in selecting employees? LO-1
-
(ii) State Wilkie's updating equation in respect of the force of inflation and explain carefully what each of the components of the equation represents. State also which type of time series process...
-
Compute the double integral D x y dA over the domain D indicated as 0 x 5, x y 2x + 3. (Use symbolic notation and fractions where needed.) f(x, y) A = D
-
4. (10 points) A researcher believes that length of time spent listening to classical music increases memory for previously learned material. She has 4 groups of 5 subjects listen to either 10 min.,...
-
We find a binary system consisting of a 1 solar mass star, still in its main sequence phase, and a white dwarf. Assume both stars formed at the same time and that they did not significantly influence...
-
The equity sections from Atticus Group's 2015 and 2016 year-end balance sheets follow. Stockholders Equity (December 31, 2015) Common stock $6 par value, 50,000 shares authorized, 35,000 shares...
-
In problem 1-5, find the limits. 1. 2. 3. 4. 5. lim x2 im lim linn (x-5)(3-x)
-
Using the theoretical sampling strategy, how many samples of size 4 (n = 4) can be drawn from a population of size: (a) N = 5? (b) N = 8? (c) N = 16? (d) N = 50?
-
Draw and name all possible aromatic Compounds with the formula C8H9Br.
-
Propose structures for aromatic hydrocarbons that meet the following descriptions: (a) C9H12; gives only one C9H11Br product on substitution with bromine (b) C10H14 gives only one C10H13C1 product on...
-
Look at the three resonance structures of naphthalene shown in Section 15.7, and account for the fact that not all carboncarbon bonds have the same length. The C1C2 bond is 136 pm long, whereas the...
-
ABC Corporation has an activity - based costing system with three activity cost pools - Machining, Setting Up , and Other. The company's overhead costs, which consist of equipment depreciation and...
-
Consolidated Balance Sheets - USD ( $ ) $ in Thousands Dec. 3 1 , 2 0 2 3 Dec. 3 1 , 2 0 2 2 Current assets: Cash and cash equivalents $ 9 8 , 5 0 0 $ 6 3 , 7 6 9 Restricted cash 2 , 5 3 2 Short -...
-
How does corporate governance contribute to investor confidence and stakeholder trust? Accounting

Study smarter with the SolutionInn App


