How would you use NMR spectroscopy to distinguish between the following pairs of compounds? (a) (b) 'N.
Question:
(a)
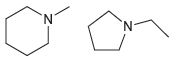
(b)
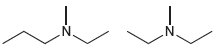
Transcribed Image Text:
'N. 'N- N.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 46% (13 reviews)
a The 1 H NMR spectrum of the first compound will have a singl...View the full answer

Answered By

Felix Mucee
I am a detailed and thorough professional writer with 5 years of administrative experience- the last 2 years in academic writing and virtual office environment. I specialize in delivering quality services with respect to strict deadlines and high expectations. I am equipped with a dedicated home office complete with a computer, copier/scanner/fax and color printer.
I provide creative and detailed administrative, web search, academic writing, data entry, Personal assistant, Content writing, Translation, Academic writing, editing and proofreading services. I excel at working under tight deadlines with strict expectations. I possess the self-discipline and time management skills necessary to have served as an academic writer for the past five years. I can bring value to your business and help solve your administrative assistant issues.
4.70+
13+ Reviews
33+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Describe how you could use NMR spectroscopy to distinguish between benzoyl chloride and para-chlorobenzaldehyde.
-
How might you use 1k spectroscopy to distinguish between the following pairs of isomers? (a) CH 4 CH 2 OH and CHOCH 3 (b) Cyclohexane and 1-hexene (c) CH 3 CH 2 CO 2 H and HOCH 2 CH 2 CHO
-
How could you use infrared spectroscopy to distinguish between the following pairs of isomers? (a) HC CCH2NH2 and CH3CH2C N (b) CH3COCH3 and CH3CH2CHO
-
A quality improvement program has been instituted in an organization to reduce total quality costs. Discuss the impact of such a program on prevention, appraisal, and failure costs.
-
A fire department keeps two rescue vehicles. Due to the demand on the vehicles and the chance of mechanical failure, the probability that a specific vehicle is available when needed is 90%. The...
-
In 2017 there was a total of 469 commercial and noncommercial orbital launches worldwide. In addition, the number of noncommercial orbital launches was 31 more than half the number of commercial...
-
Who is intended to be protected by minimum legal capital? AppendixLO1
-
Table 6-12 on the textbook's Web site gives non seasonally adjusted quarterly data on the retail sales of hobby, toy, and game stores (in millions) for the period 1992:1 to 2008: II. Consider the...
-
i need help correct if I'm wrong answer in order !! I need help with Memo partt !! Here's Memo Part correct if I'm wrong !! Default (Ca. M A1 100% $ 0.00 123 fx Traditional Costing E Traditionhl...
-
Consider the window size is 10, bandwidth is 1500 bps, transmission delay is 2 ms and propagation delay is 50 ms. What is the throughput using Go Back N protocol?\\ a) 294.11\\ b) 140.23\\ c) 96\\ d)...
-
Evaluate the screening used to qualify the respondents. Are there any changes needed? Why or why not?
-
Given the information on sampling, were there any errors? What would you have done differently?
-
Nitrogen, N 2 , can ionize to form N 2 + or add an electron to give N 2 . Using molecular orbital theory, compare these species with regard to (a) Their magnetic character, (b) Net number of bonds,...
-
1. A corn farmer has observed the following distribution for the number of ears of corn per cornstalk. Ears of Corn Probability 1 2 3 4 .3 .4 .2 .1 Part A: How many ears of corn does he expect on...
-
1. A mass m on a vertical spring with force constant k has an amplitude of A. Using the top of the motion as the origin for both gravitational potential energy and spring potential energy: (a) Find...
-
2. Consider the PDE Utt - Uxx + Ut - Ux = 0 (1) for < < and 0
-
On April 1, 2024, Chardonnay pays an insurance company $12,480 for a two- year fire insurance policy. The entire $12,480 is debited to Prepaid Insurance at the time of the purchase. Record the...
-
Which retailer(s) should represent and sell your product?Why?In terms of their range of distribution coverage, is your retailer intensive, selective and exclusive? Why is this aspect important to...
-
In Exercises 119120, solve and graph the solution set on a number line. 3|2x + 6-9 < 15
-
The graph of the sequence whose general term is an = n - 1 is which of the following? [8.1] A. B. TITTT 3-2-1 23.45 2.3.4
-
Trans-1, 2-Dimethylcyclobutane is more stable than cis-1, 2-dimethylcyclobutane, but cis-1, 3-dimethylcyclobutane is more stable than trans-1, 3-dimethylcyclobutane. Use drawings to explain these...
-
Name the following alkanes and haloalkanes. When two or more substituents are present, list them in alphabetical order. (a) (b) (c) (d) CH CH CH-CH CHCH Br CH CH CH CH CH CH CH CH, CH,CH(CH3)....
-
The cyclohexane chair just drawn has the headrest to the left and the footrest to the right. Draw a cyclohexane chair with its axial and equatorial bonds, having the headrest to the right and the...
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


