In the unimolecular isomerization of cyclobutane to butylene, the following values for kuni as a function of
Question:
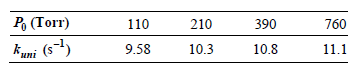
Assuming that the Lindemann mechanism accurately describes this reaction, determine k1 and the ratio k€“1/k2
Transcribed Image Text:
390 210 110 Po (Torr) 760 11.1 10.8 10.3 9.58 kuni (s-)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
The Lindemann mechanism predicts the following relationship between the unimol...View the full answer

Answered By

Allan Simiyu
I am an adroit Writer. I am a dedicated writer having worked as a writer for 3 years now. With this, I am sure to ace in the field by helping students break down abstract concepts into simpler ideas.
5.00+
8+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the unimolecular isomerization of methylcyanide, a reaction that will be discussed in detail in Chapter 36: CH 3 NC(g) CH 3 CN(g) The Arrhenius parameters for this reaction are A = 2.5 10...
-
In Problem 22.10 the isomerization of cyclopropane over a limited pressure range was examined. If the Lindemann mechanism of first order reactions is to be tested we also need data at low pressures....
-
The isomerization of cyclopropane, C3H6, to propylene, CH2=CHCH3, is first order in cyclopropane and first order overall. At 1000oC, the rate constant is 9.2/s. What is the half-life of cyclopropane...
-
k) Assume that one of these portfolio's is the Market Portfolio and all portfolios, except Portfolio G, are fairly priced according to the CAPM. What is the highest utility score that can be achieved...
-
Prove that if f is a one-to-one odd function, then f1 is an odd function.
-
Show that the Lagrangian L(r, v) = mc 2 / + ev A(r, t) e(r, t) predicts the correct relativistic equation of motion for a point particle with mass m and charge e.
-
If the forecast for February was 122 and actual demand was 135, what would be the forecast for March if the smoothing constant () is 0.15? Use exponential smoothing for your calculation. LO.1
-
Pink Martini Company has gathered the following information. Units in beginning work in process ............20,000 Units started into production .............72,000 Units in ending work in process...
-
Shadee Corporation expects to sell 5 9 0 sun shades in May and 4 3 0 in June. Each shade sells for $ 1 4 3 . Shadee's beginning and ending finished goods inventories for May are 8 5 and 4 5 shades,...
-
According to Money magazine, Maryland had the highest median annual household income of any state in 2018 at $75,847 (Time.com website). Assume that annual household income in Maryland follows a...
-
The chlorination of vinyl chloride, C 2 H 3 Cl + Cl 2 C 2 H 3 Cl 3 , is believed to proceed by the following mechanism: Derive the rate law expression for the chlorination of vinyl chloride based on...
-
In the discussion of the Lindemann mechanism, it was assumed that the rate of activation by collision with another reactant molecule, A, was the same as collision with a nonreactant molecule, M, such...
-
Geek Squad performs services for a customer and bills the customer for $500. How would Geek Squad record this transaction? a. Accounts receivable increase by $500; revenues increase by $500. b. Cash...
-
Sunn Company manufactures a single product that sells for $180 per unit and whose variable costs are $141 per unit. The company's annual fixed costs are $636,000. The sales manager predicts that next...
-
Question 22(5 points) Silver Corp. declares a 15% stock dividend to its shareholders on 1/18. On that date, the company had 15,000 shares issued and 12,000 shares outstanding. Silver Corp. common...
-
Select your a diagnosis from the DSM-5. using your information found through a search of the literature available on your selected diagnosis using appropriate references of peer reviewed journal...
-
Problem 1: Grand Monde Company manufactures various lines of bicycles. Because of the high volume of each type of product, the company employs a process cost system using the FIFO method to determine...
-
Draw the shear force, bending moment diagram of a beam for the loading condition as shown in the figure. Determine the maximum bending moment, and shear force in the beam. Support reactions are pre-...
-
If the graph of y = f(x) undergoes a horizontal stretch or shrink to become the graph of y = g(x). do these two graphs have the same x-intercepts? y-intercepts? Explain your answers.
-
Activator rod AB exerts on crank BCD a force P directed along line AB. Knowing that P must have a 100-N component perpendicular to arm BC of the crank, determine (a) The magnitude of the force P, (b)...
-
Calculate the average CH bond enthalpy in methane using the data tables. Calculate the percent error in equating the average CH bond energy in Table 4.3 with the bond enthalpy. Table 4.3 Selected...
-
The heat capacity of α -quartz is given by The coefficient of thermal expansion is given by β = 0.3530 à 10 4 K 1 and V m = 22.6 cm 3 mol+. Calculate ÎS m for...
-
The following cyclic ether can be prepared via an intramolecular Williamson ether synthesis. Show what reagents you would use to make this ether.
-
Los datos de la columna C tienen caracteres no imprimibles antes y despus de los datos contenidos en cada celda. En la celda G2, ingrese una frmula para eliminar cualquier carcter no imprimible de la...
-
Explain impacts of changing FIFO method to weighted average method in inventory cost valuations? Explain impacts of changing Weighted average method to FIFO method in inventory cost valuations?...
-
A perpetuity makes payments starting five years from today. The first payment is 1000 and each payment thereafter increases by k (in %) (which is less than the effective annual interest rate) per...

Study smarter with the SolutionInn App


