Answered step by step
Verified Expert Solution
Question
1 Approved Answer
50 mL Non-Aqueous Layer 20 mL Aqueous Layer Volume of 0.0500M HCl (mL [NH 3 ] c (M) Volume of 0.500M HCl (mL) Total [NH
| 50 mL Non-Aqueous Layer | 20 mL Aqueous Layer | |||
|---|---|---|---|---|
| Volume of 0.0500M HCl (mL | [NH3]c (M) | Volume of 0.500M HCl (mL) | Total [NH3] (M) | n |
| 33.5 | ? | 21.2 | ? | ? |
| 25.1 | ? | 20.1 | ? | ? |
| 22.7 | ? | 22.37 | ? | ? |
By given the following information:
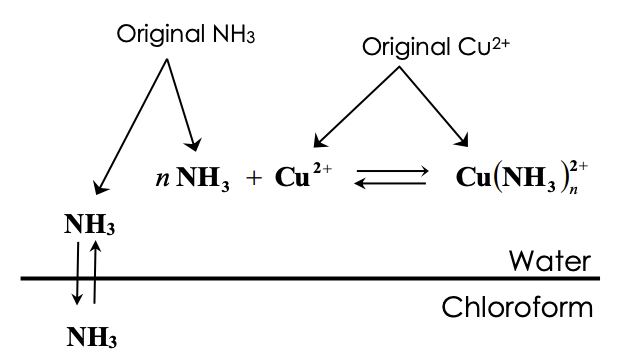
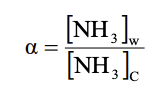
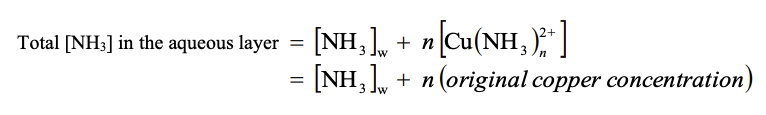

where the original copper concentration = 0.5 M
Find the values for all "?" in the table. (if the calculation steps are similar, just one well-explained sample calculation is enough, thank you)
Original NH3 Original Cu2+ NH3 =[NH3]C[NH3]w Total[NH3]intheaqueouslayer=[NH3]w+n[Cu(NH3)n2+]=[NH3]w+n(originalcopperconcentration) n=(originalcopperconcentration)total[NH3],aqueouslayer[NH3]cStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


