Amino acids can be used as enantiomerically pure starting materials in organic synthesis. Scheme I depicts the
Question:
Amino acids can be used as enantiomerically pure starting materials in organic synthesis. Scheme I depicts the first steps in the synthesis of a reagent employed in the preparation of enantiomerically pure β-amino acids, such as that occurring in the side chain of taxol. Scheme II features an ester of the same amino acid for the preparation of an unusual heterocyclic dipeptide used in the study of polypeptide conformations.
Scheme I: Synthesis of an enantiomerically pure reagent
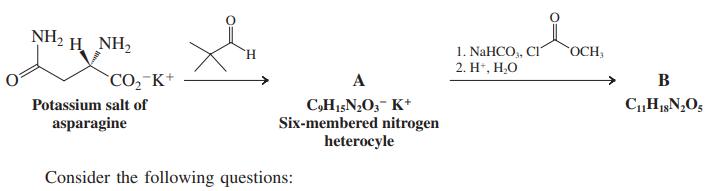
Consider the following questions:
1. There are two diastereomers of A formed in a 90 : 10 ratio. The major isomer is the one that can attain the most stable chair conformation. Are the two substituents on the ring cis or trans to each other? Label their positions as equatorial or axial.
2. Which nitrogen is nucleophilic and yields the carbamic ester B?
Scheme II: Synthesis of an unusual heterocyclic dipeptide
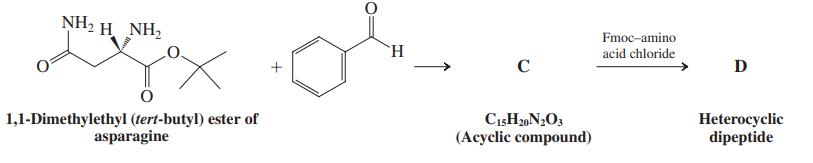
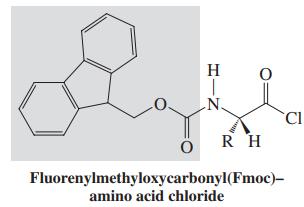
Consider the following questions:
1. What functional group is generated in C?
2. Where is the peptide bond in D? Circle it.
Reconvene to discuss the answers to the questions posed in each scheme and the structures you proposed for A – D.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





