Structure A (shown in the margin) is that of a naturally occurring a-amino acid. Select its name
Question:
Structure A (shown in the margin) is that of a naturally occurring a-amino acid. Select its name from the following list.
(a) Glycine;
(b) Alanine;
(c) Tyrosine;
(d) Cysteine.
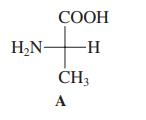
Transcribed Image Text:
СООН H,N- -H- CH3 A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Structure A Answer b Alanine Structure A is that of a nat...View the full answer

Answered By

Vinod Antil
I had completed my graduation (BSc Hons chemistry)from University of Delhi in2015
I am pg holder. I had completed my post graduation (MDU , Rohtak)in 2017.
I has qualified CSIR JRF NET exam and Gate exam.
From 2018 March onwards I am giving the sevice in Eureka public school ,Narnaul.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
When compound A (shown in the margin) is treated with dilute mineral acid, an isomerization takes place. Which of the following compounds is the new isomer formed? CHCH3 CHCH3 A
-
Amino acid analysis of a certain tetrapeptide gave alanine, glycine, phenylalanine, and valine in equimolar amounts. What amino acid sequences are possible for this tetrapeptide?
-
Most naturally occurring amino acids have chirality centers (the asymmetric carbon atoms) that are named (S) by the Cahn-Ingold-Prelog convention (Section 5-3). The common naturally occurring form of...
-
Troy Engines, Ltd., manufactures a variety of engines for use in heavy equipment. The company has always produced all of the necessary parts for its engines, including all of the carburetors. An...
-
In 1985, neither Florida nor Georgia had laws banning open alcohol containers in vehicle passenger compartments. By 1990, Florida had passed such a law, but Georgia had not. (i) Suppose you can...
-
The latitude and longitude of a point P in the Northern Hemisphere are related to spherical coordinates p, , as follows. We take the origin to be the center of the earth and the positive z-axis to...
-
1 Relate these accounts to the stages of team development.
-
Tablon Inc. is a wholly owned subsidiary of Marbel Co. The philosophy of Marbel's management is to allow the subsidiaries to operate as independent units. Corporate control is exercised through the...
-
6. For the following below, determine the after-tax weighted average cost of debt: The tax rate is 35% A. Loan A= $250 @ 11% Loan B = $750 @ 4% B. Loan A $200 @ 5% Loan B $800 @ 7% Loan C $1,000 @...
-
The Piedmont Investment Corporation has identified four small apartment buildings in which it would like to invest. The four banks generally used by Piedmont have provided quotes on the interest...
-
Amino acids can be used as enantiomerically pure starting materials in organic synthesis. Scheme I depicts the first steps in the synthesis of a reagent employed in the preparation of...
-
The primary structure of a protein refers to: (a) Cross-links with disulfide bonds; (b) Presence of an a helix; (c) The a-amino acid sequence in the polypeptide chain; (d) The orientation of the side...
-
Fair Value Hedge: Long in Commodity Futures Daley, Inc., engages in futures trading on a regular basis. Its books are closed on June 30 of each year. On June l, 2014, Daley enters a futures contract...
-
z = 1.1 for H a : < 149.6 Find the P-value that corresponds to the standard z-score, and determine whether the alternative hypothesis is supported at the 0.05 significance level.
-
An object is placed \(150 \mathrm{~mm}\) away from a converging thin lens that has a focal length of \(400 \mathrm{~mm}\). What are (a) the image distance and \((b)\) the magnification? (c) Draw a...
-
Let $M$ be the four-dimensional Minkowski space, with coordinates $x^{0}, x^{1}, x^{2}$, and $x^{3}$. Let us define a linear operator $*: \Omega^{r}(M) ightarrow$ $\Omega^{4-r}(M)$, such that...
-
You must select an orifice meter for measuring the flow rate of an organic liquid ( $\mathrm{SG}=0.8$, $\mu=15 \mathrm{cP}$ ) in a $4 \mathrm{in}$. sch 40 pipe. The maximum flow rate anticipated is...
-
A team of designers was given the task of reducing the defect rate in the manufacture of a certain printed circuit board. The team decided to reconfigure the cooling system. A total of 973 boards...
-
Suppose that we want to compute the geometric mean of a list of positive values. To compute the geometric mean of k values, multiply them all together and then compute the kth root of the value. For...
-
A city maintains a solid waste landfill that was 12 percent filled at the end of Year 1 and 26 percent filled at the end of Year 2. During those periods, the government estimated that total closure...
-
Reaction of HBr with 2-methyipropenc yields 2-hromo-2-methylpropane, what is the structure of the carbocation formed during the reaction? Show the mechanism of the reaction. CH CHBr C=CH2 + HBr H...
-
Add curved arrows to the following polar reactions to indicate the flow of electrons in each: :Ci: (a) - :- + -N :CI: -NH (b) H CH: -Br: :Br: (c) :0: :CI: CI "
-
Predict the products of the following polar reaction, a step in the citric acid cycle for food metabolism, by interpreting the flow of electrons indicated by the curve darrows: H2 CO2 Lc-co, -0,C-CH2
-
Time Value of Money: Basics Using the equations and tables in Appendix 12A of this chapter, determine the answers to each of the following independent situations. Round answers to the nearest whole...
-
Compute the cost assigned to ending inventory using (a) FIFO, (b) LIFO, (c) weighted average, and (d) specific identification. For specific identification, the March 9 sale consisted of 45 units from...
-
Inventory Inventory Quantity Cost per Unit Item Market Value per Unit (Net Realizable Value) Birch $85 Cypress $69 112 137 Mountain Ash 183 207 Spruce 210 188 Willow 279 Inventory at the Lower of...

Study smarter with the SolutionInn App


