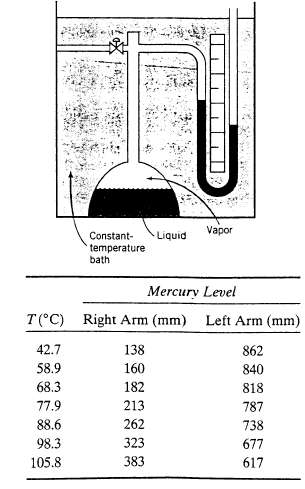
The apparatus shown here is used to measure the vapor pressure of ethylene diamine. The system is
Question:
The apparatus shown here is used to measure the vapor pressure of ethylene diamine. The system is loaded with pure ethylene diamine and the bath is adjusted to each of several known temperatures. The following readings are taken on a day when the atmospheric pressure is 758.9 mm Hg:
(a) Calculate p for ethylene diamine at each temperature.
(b) Use a semi log plot of p* versus 1/T to estimate the normal boiling point and the heat of vaporization of ethylene diamine.
(c) Does the Clausius?Clapeyron equation appear to be justified for ethylene diamine in the temperature range covered by the data? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:





