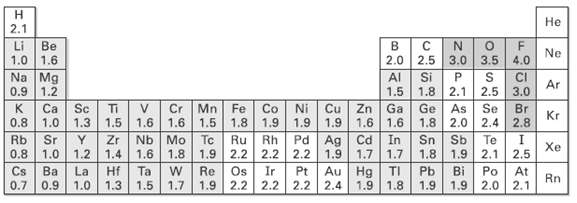
Use the electro-negatively values shown in Figure to rank the following bonds from least polar to most
Question:
Use the electro-negatively values shown in Figure to rank the following bonds from least polar to most molar: H3C ? Li, H3C ? K, H3C ? F, H3C ? MgBr, H3C ? OH.
When companies need to raise money, issuing bonds is one way to do it. A bond functions as a loan between an investor and a corporation. The investor agrees to give the corporation a specific amount of money for a specific period of time in exchange...
Transcribed Image Text:
Не 2.1 Li Be 1.0 1.6 Na Mg 0.9 1.2 Ca 0.8 Ne 4.0 2.0 2.5 3.0 3.5 Al 1.5 Si 1.8 2.1 2.5 Ge As 1.8 2.0 2.4 CI Ar 3.0 Mn Fe V Cr 1.6 1.5 1.8 Sc Ti 1.3 Ga Cu Zn 1.6 Br Se Ni Co 1.9 Kr 2.8 1.0 Rb 1.5 1.9 Rh Pd 2.2 2.2 1.9 1.6 1.6 In Ag Cd 1.7 Zr Nb Mo| Tc Ru 1.6 Sr 0.8 Sb Sn 1.9 2.1 Xe Te 2.5 1.0 1.2 1.4 La Hf 1.0 1.3 1.5 1.8 2.2 Re Os 1.9 2.2 1.7 1.9 1.9 1.7 1.8 Bi Pb Po 1.9 1.9 2.0 Au Hg Ba Cs 0.7 TI At Rn 2.1 Ta Ir Pt 2.2 2.2 2.4 1.9 1.8 0.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 91% (12 reviews)
Strategy Use Figure to find the electronegativities of each element Calcula...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Use the electro negatively table (Figure) to predict which bond in each of the following sets is more polar, and indicate the direction of bond polarity for each compound. (a) H3C ? C1 OR C1 ? C1 (b)...
-
Modify the diagram shown in Figure to include feed forward command compensation with a constant compensator gain Kf. Determine whether such compensation can eliminate steady-state error for step and...
-
An engineer has proposed the circuit shown in figure to filter out high-frequency noise. Determine the values of the capacitor and resistor to achieve a 3-dB voltage drop at 23.16kHz. ww R
-
What have researchers found about the use of job costing to record the cost of handproducing a bound book?
-
Harriet, an organic farmer, has owned depreciable farm equipment for several years. Is the equipment a capital asset? Why or why not?
-
1. What strategic reasons could Tata Motors have for acquiring Jaguar Land Rover? 2. Evaluate Fords decision to sell Jaguar Land Rover. 3. What lessons can be learned about acquiring companies from...
-
We suspect that kids who sext (send nude photos by text) text more than kids who dont sext. Using the PewKids dataset, get 95% confidence intervals that will help you address this suspicion. Based on...
-
Purchasing Survey asked purchasing professionals what sales traits impressed them most in a sales representative. Seventy-eight percent selected "thoroughness." Forty percent responded "knowledge of...
-
Makani Handcraft is a manufacturer of picture frames for large retailers. Every picture frame passes through two departments: the assembly department and the finishing department. This problem...
-
Consider a scenario in which Host A wants to simultaneously send packets III Hosts Band C. A is connected to Band C via a broadcast channel-a packet sent by A is carried by the channel to both Band...
-
Use the + / convention to show the direction of expected polarity for each of the following bonds indicated. (a) H3C C1 (b) H3C NH2 (c) H2N H (d) H3C SH (e) H3C MgBr (f) H3C F
-
Look at the following electrostatic potential map of chloromethane, and tell the direction of polarization of the C ? C1 bonds: CI C-H Chloromethane H.
-
A 22-cm-diameter bowling ball has a terminal speed of 77 m/s. What is the ball's mass?
-
1. The interest rate charged on a loan of $85,000 is 7.75% compounded annually. If the loan is to be paid off over seven years, calculate the size of the annual payments. 2. A $10,000 debt is repaid...
-
Referring to the NISSAN Navara 5L SE M/T, which is using Nissan YD25 engine, provide your analysis to the following questions: A. What will be the maximum power produced by this vehicle at the...
-
Convert each pair of rectangular coordinates to polar coordinates where r> 0 and 0 <2.
-
Please type your answers and submit them on Blackboard by the due time. AAA corp. had the following PP&E values on Dec. 31, 2018. Cost $ 100 Accumulated Depreciation $ 20 Undiscounted Future Cash...
-
Compute the standard deviation"sigma symbol"for ages of British nurses in 1851. Assume that the table below shows the age distribution of nurses in Great Britain in 1851. Round your answer to nearest...
-
Which two statements are least appropriate? In terms of taking internal action against an employee, there are certain principles that should be followed: a. Investigate and gather the facts carefully...
-
Explain the circumstances that could result in a long-term bank loan being shown in a statement of financial position as a current liability.
-
The following data are available for the thermodynamic properties of graphite and diamond: Assuming that the entropies and densities are approximately independent of temperature and pressure,...
-
Arrange these compounds in order of increasing acid strength: CH3COH .. HOC-COH HOCCH2CH2COH
-
Use the tables in this chapter to predict whether these equilibria favor the reactants or the products: CHH CH + CHCH-N-CHCH, NHCHC=C: + :NH c) CHC=C-H+ (CH3)3C-0: Dc0: CH3 CH3 + CHCH,CHCH, +...
-
Complete these equilibrium reaction sin the most reasonable manner possible using the curved arrow convention to show the movement of electrons in the reactions, Predict whether the reactants or the...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


