A reaction was believed to occur by the following mechanism. (a) Write the overall reaction. (b) Write
Question:
A reaction was believed to occur by the following mechanism.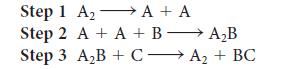
(a) Write the overall reaction.
(b) Write the rate law for each step and indicate its molecularity.
(c) What are the reaction intermediates?
(d) A catalyst is a substance that accelerates the rate of a reaction and is regenerated in the process. Which substance is functioning as a catalyst in the reaction?
Transcribed Image Text:
Step 1 A₂ A + A → Step 2 A + A + B A₂B Step 3 A₂B + CA₂+ BC
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a The overall reaction can be written as A2 2B C 2A BC b The rate law for each s...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The following mechanism has been proposed for the gasphase reaction between HBr and NO 2 : (a) Write the overall reaction. (b) Write the rate law for each step and indicate its molecularity. (c) What...
-
The reaction I2(aq) + OCl2(aq) IO2(aq) + Cl2(aq) is believed to occur by the following mechanism: Write the rate law for this reaction.
-
The isomerization of cyclopropane, C3H6, is believed to occur by the mechanism shown in the following equations: Here C3H6* is an excited cyclopropane molecule. At low pressure, Step 1 is much slower...
-
Consider a random walk consisting of equi-probable p = q = 1/2 steps in left or right directions. However the step length at ith step is given by e-, i = 1,2,3..... N, with > 0 a constant. Calculate...
-
Contemporary computers often have more than 100MB of physical memory. Suppose the page size is 2KB. How many entries would an associative memory need in order to implement a page table for the memory?
-
An object lying on Earth's equator is accelerated (a) Toward the center of Earth because Earth rotates, (b) Toward the Sun because Earth revolves around the Sun in an almost circular orbit, and (c)...
-
Describe the functional strategies an organization needs and explain how those strategies are implemented and evaluated. lo1
-
Your client, Red Horse Inc., prepared the following schedule for long term debt for the audit of financial statements for the year ended December 31, 2011: Required a. What type of evidence would you...
-
Assume that a company is pursuing a strategy based on product leadership. Which if-then hypothesis statement is most aligned with this strategy rather than an operational excellence or customer...
-
The Jackson independent School District began the year with the following accounts on its Balance Sheet related to property taxes (all amounts are in thousands of dollars). All accounts have normal...
-
Substance A decomposes in a first-order reaction and its half life is 355 s. How much time must elapse for the concentration of A to decrease to (a) One-eighth of its initial concentration; (b)...
-
The decomposition of gaseous dinitrogen pentoxide in the reaction 2 N 2 O 5 (g) 4 NO 2 (g) + O 2 (g) gives the data shown here at 298 K. (a) Using a graphing calculator or standard graphing software,...
-
The Transcontinental Trucking Company wishes to route a shipment from Buffalo to Duluth over major highways. Because time and distance are closely related, the company dispatcher would like to find...
-
A liquid mixture of 65 mole% n-nonane and 35 mol% n-octane enters a flash unit. In the flash unit, the pressure is reduced to 1 atm and half of the liquid is evaporated. find the temperature in the...
-
To gain a deep understanding of SAPPI LIMITED's industry and competitive environment, answer the following questions before the company can embark on a "new strategy" breakaway. Does this industry...
-
What communication tools can a manager use to construct and deliver constructive and timely feedback to their employees? Discuss the various communication tools (i.e. email, phone, text, social...
-
The production per hour, output per unit time, and the number of operations per hour are all examples of labor standards (David & Davis, 2020). Employees will experience both good and negative...
-
Explain in detail on the following CLO 5 -Evaluate strategic implementation and control principles/improvement strategies for business control, including use of strategic Dashboards and Balance...
-
The following is a sample of computer output from a study. Describe the problem and the conclusion, based on the computer output. Y = number of drinks in the previous 7 days Two-sample T for...
-
Which of the following streaming TV devices does not involve use of a remote controller? A) Google Chromecast B) Apple TV C) Amazon Fire TV D) Roku
-
In the presence of a special type of catalyst, hydrogen gas will add across a triple bond to produce a double bond: The process is exothermic. Do you expect a high temperature to favor products or...
-
Consider the following reaction. Predict whether an increase in temperature will favor reactants or products. Justify your prediction. +
-
When an amine is protonated, the resulting ammonium ion is not electrophilic: However, when an imine is protonated, the resulting iminium ion is highly electrophilic: Explain this difference in...
-
During 2024, its first year of operations, Hollis Industries recorded sales of $11,900,000 and experienced returns of $760,000. Cost of goods sold totaled $7,140,000 (60% of sales). The company...
-
What is the value of a 15% coupon bond with 11% return? Is it a discount or a premium bond?
-
A manufacturer with a December 31 taxation year end sells new machinery for $50,000 on January 2, 2022. The cost of the machinery is $20,000. The terms of the sale require an initial payment of...

Study smarter with the SolutionInn App


