For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or
Question:
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: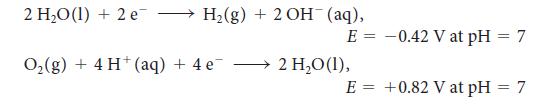 When a ruthenium chloride solution was electrolyzed for 500 s with a 120-mA current, 31.0 mg of ruthenium was deposited. What is the oxidation number of ruthenium in the ruthenium chloride?
When a ruthenium chloride solution was electrolyzed for 500 s with a 120-mA current, 31.0 mg of ruthenium was deposited. What is the oxidation number of ruthenium in the ruthenium chloride?
Transcribed Image Text:
2 H₂O(1) + 2 e → H₂(g) + 2 OH¯ (aq), E = O₂(g) + 4 H (aq) + 4 e 2 H₂O (1), E = -0.42 V at pH = 7 +0.82 V at pH = 7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: Thomas Edison was faced...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A sample of manganese of...
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: A 1.0 m KBr(aq) solution...
-
Consider the following loan information. . Total acquisition price: $3,000,000. Property consists of twelve office suites, five on the first floor and seven on the second. Contract rents: three...
-
Allocating costs of support departments; step-down and direct methods, the Central Valley Company has prepared department overhead budgets for budgeted-volume levels before allocations as follows:...
-
Is a high-performance transaction system necessarily a real-time system? Why or why not?
-
Pallos Company is purchasing the net assets of Shrilly Company. The book and fair values of Shrillys accounts are as follows: Accounts Book Fair Current assets . . . . . . . . . . . . . . . . . . . ....
-
Hixson paid Galyen Petroleum Co. money he owed by issuing three checks to Galyen. The bank refused to cash the three checks because of insufficient funds in the Hixson account to pay all three....
-
What is the present value of $8,000 paid at the end of each of the next 24 years if the interest rate is 9% per year
-
A commercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at -35C by rejecting waste heat to cooling water that enters the condenser at 18C at a rate...
-
A lead electrode in 0.020 m Pb(NO 3 ) 2 (aq) is connected to a hydrogen electrode in which the pressure of H 2 is 1.0 bar. If the cell potential is 0.078 V at 25C, what is the pH of the electrolyte...
-
Calculate the pH of the solution that results from mixing (a) 0.100 L of 0.050 m (CH 3 ) 2 NH(aq) with 0.280 L of 0.040 m (CH 3 ) 2 NH 2 Cl(aq); (b) 45.0 mL of 0.015 m (CH 3 ) 2 NH(aq) with 86.0 mL...
-
Consider a company that pays out all its earnings. The required return for the firm is 13 percent. a. Compute the intrinsic P/E value of the company if its ROE is 15 percent. b. Compute the intrinsic...
-
The current rate of interest on S-T Treasury Bills = 10%, intermediate term Gov. Bonds = 11%, Lt- Gov. Bonds = 12%, AA rate Corp. Bonds = 13.5% and the rate of inflation is 5%. Holding-period returns...
-
Prepare Income Statement(absorption costing) for the second, third and fourth month. SALES (SP X unit sold) INCOME STATEMENT FORMAT (ABSORPTION COSTING) XXX Less: Cost of Goodsold VARIABLE COST (VC...
-
The following shows the distribution of final exam scores in a large introductory psychology class. The proportion under the curve is given for two segments (short answers-no calculations required)....
-
How much overhead was included in the cost of Job #461 at the beginning of January? * (1 Point). BREAD Co. uses a job order costing system. At the beginning of January, the company had 2 jobs in...
-
3. (3pt.) A state of a physical system is just a description of the system at an instant in time in terms of its properties. In classical mechanics, states are represented by points (in phase space)....
-
For each of the following cases (a and b), (i) state whether the study should be observational or experimental and why. (ii) state whether blinding should be used. If the study should be run blind or...
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
Two liquids, A and B, are immiscible for T < 75.0C and for T > 45.0C and are completely miscible outside of this temperature range. Sketch the phase diagram, showing as much information as you can...
-
At 350. K, pure toluene and hexane have vapor pressures of 3.57 10 4 Pa and 1.30 10 5 Pa, respectively. a. Calculate the mole fraction of hexane in the liquid mixture that boils at 350. K at a...
-
The partial molar volumes of water and ethanol in a solution with xH 2 O = 0.45 at 25C are 17.0 and 57.5 cm 3 mol 1 , respectively. Calculate the volume change upon mixing sufficient ethanol with...
-
Question 3 (24 marks) Wonderful Technology Company Limited sells computers and accessories. Data of the store's operations are as follow: Sales are budgeted at $400,000 for December 2019, $420,000...
-
Kratz Manufacturing Company uses an activity-based costing system. It has the following manufacturing activity areas, related cost drivers and cost allocation rates: Activity Cost Driver Cost...
-
You are a Partner with Fix-It Consultants and have been engaged in an advisory capacity with a software company, called MoveFast. The company is seeing a sharp decline in revenue, with the primary...

Study smarter with the SolutionInn App


