The accompanying table provides the identity of the two naturally occurring isotopes for four elements and theatomic
Question:
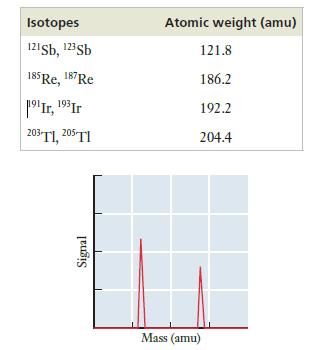
The accompanying table provides the identity of the two naturally occurring isotopes for four elements and the atomic weights for those elements. (In each case, the two isotopes differ in mass number by two.) Which element has the mass spectrum shown? Explain your answer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





