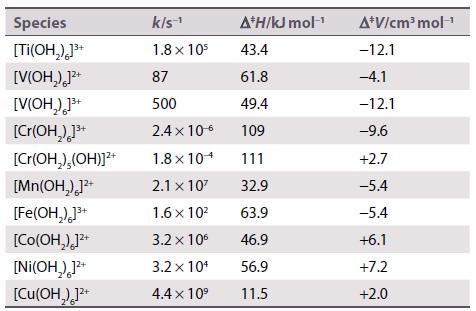
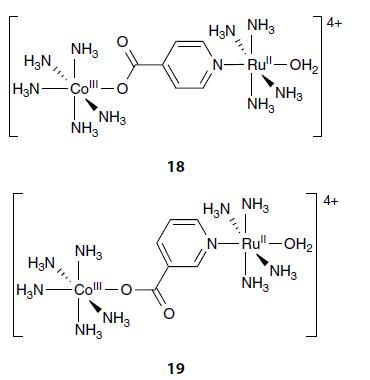
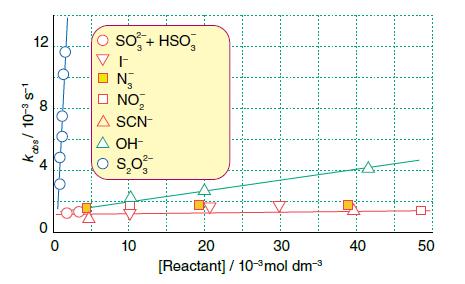
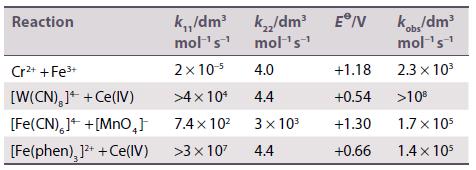
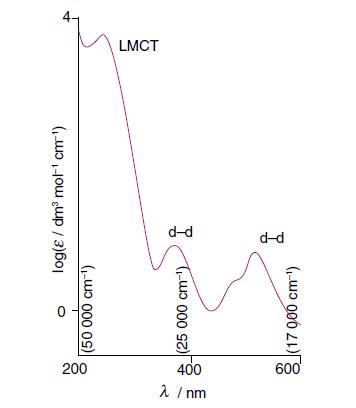
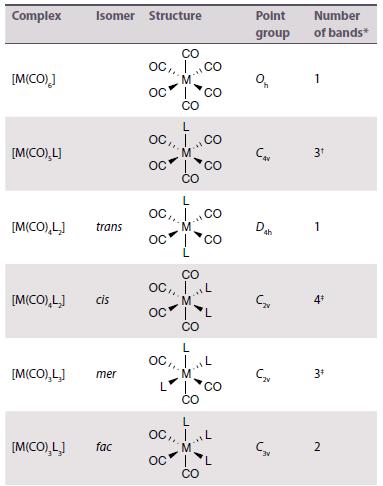
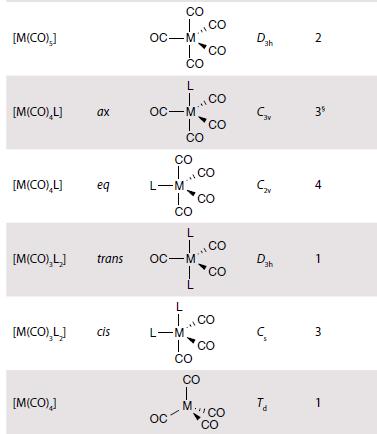
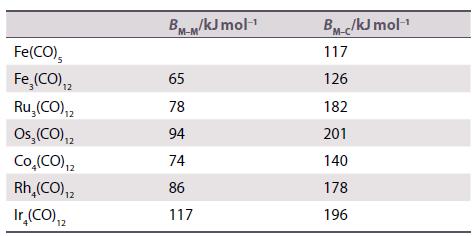
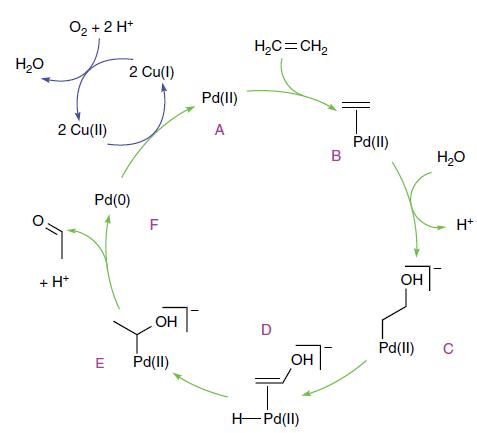
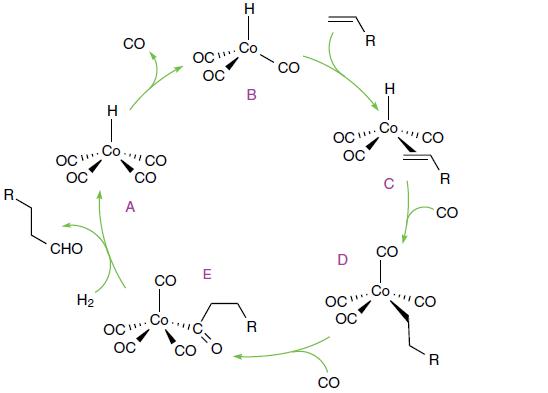
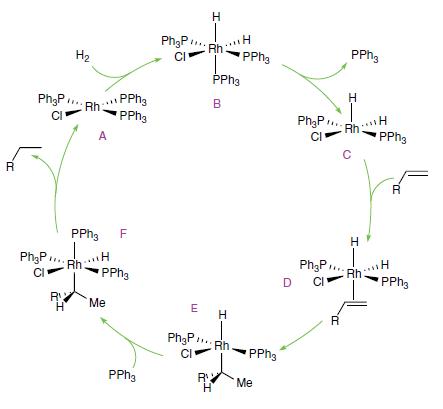
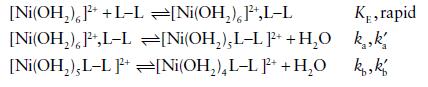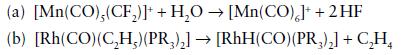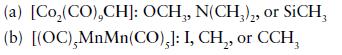Inorganic Chemistry 7th Edition Mark Weller, Tina Overton, Jonathan Rourke - Solutions
Discover comprehensive support for "Inorganic Chemistry 7th Edition" by Mark Weller, Tina Overton, and Jonathan Rourke. Access our Online platform for a complete Answers key and Solution manual, featuring detailed solutions and a Solutions PDF for seamless learning. Tackle solved problems with our questions and answers, ensuring a deeper understanding of the textbook's concepts. Enhance your study sessions with our Test bank and Chapter solutions, providing Step by step answers to complex queries. Benefit from the instructor manual for guided learning. Enjoy free download options to make your textbook journey effortless and enriching.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()