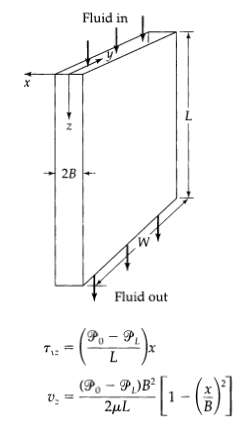
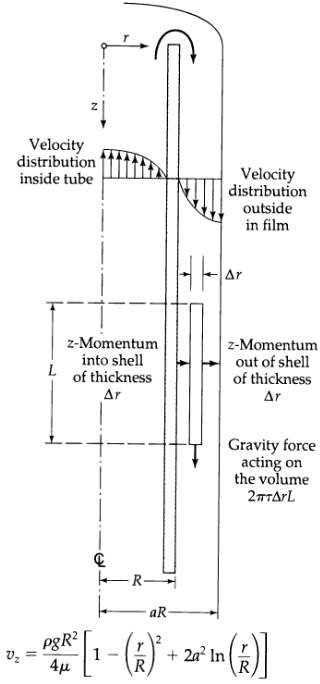
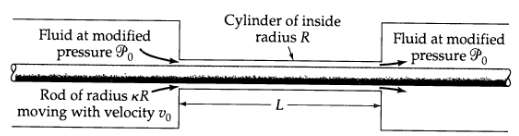
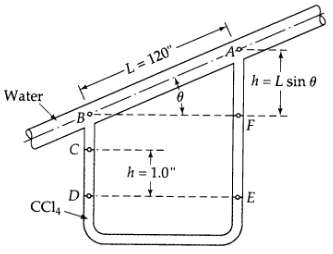
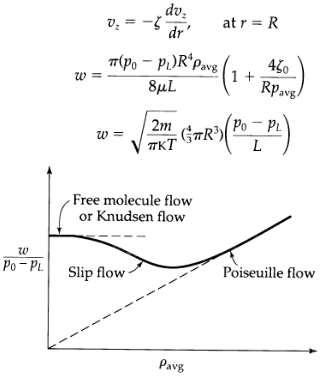
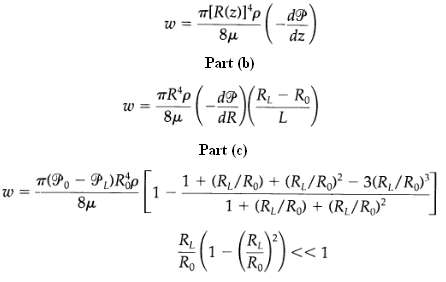
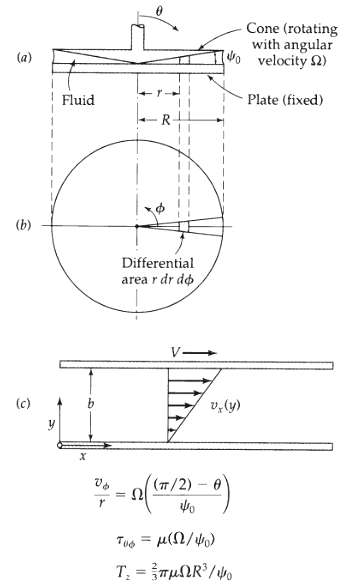
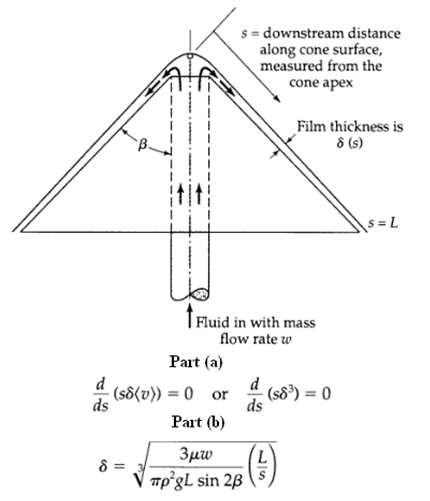
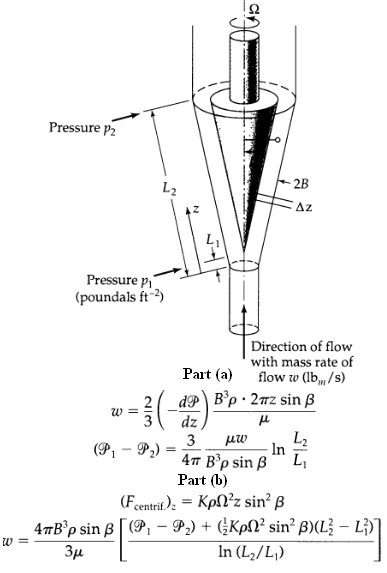
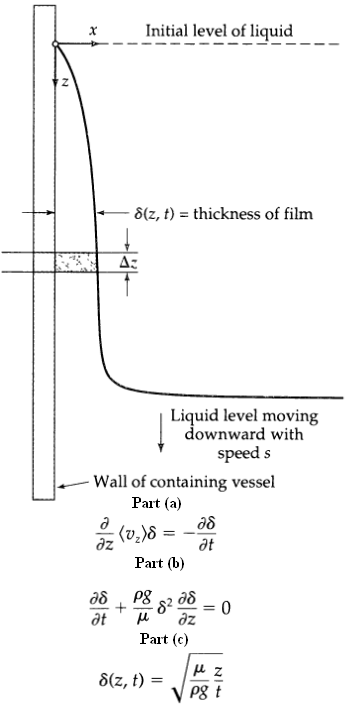
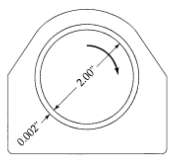
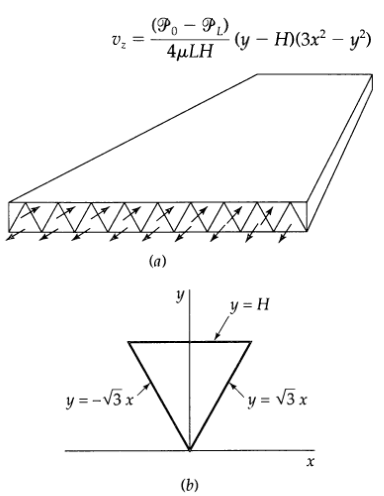
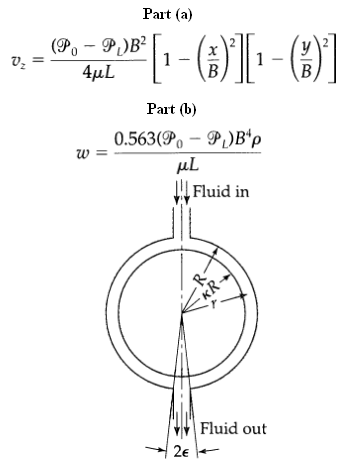
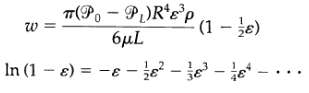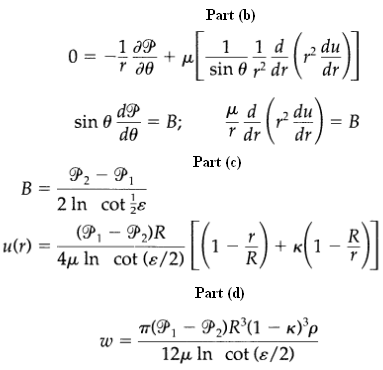Transport Operations 2nd Edition Allen Stuart - Solutions
Discover the comprehensive solutions for "Transport Operations 2nd Edition" by Allen Stuart with our detailed answers key and solutions manual. Access the complete solutions PDF featuring step-by-step answers to all solved problems. Enhance your understanding with our test bank, chapter solutions, and instructor manual, making this textbook an invaluable resource for both students and instructors. Enjoy the convenience of online access and the ability to free download these questions and answers. Unlock the full potential of your studies with our expertly crafted solutions, tailored for a seamless learning experience.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()