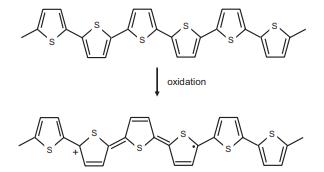
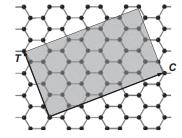
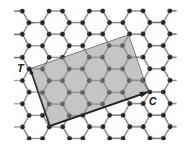
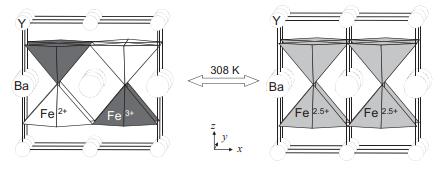
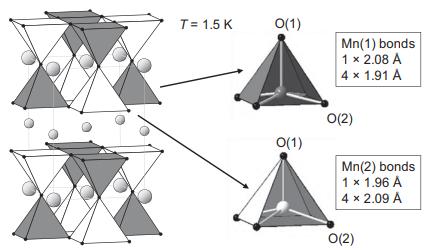
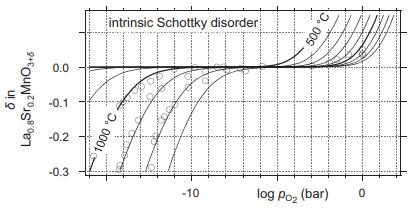
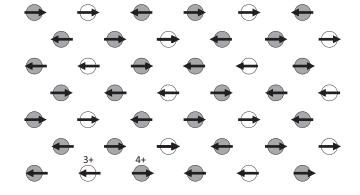
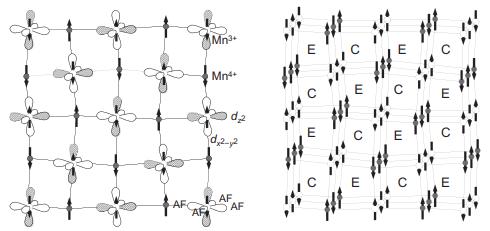
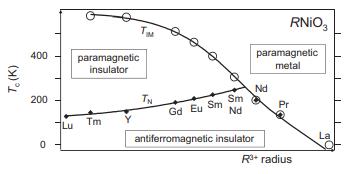
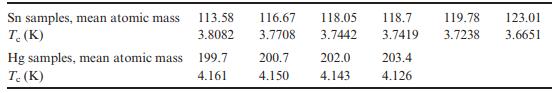
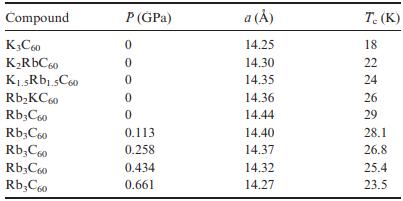
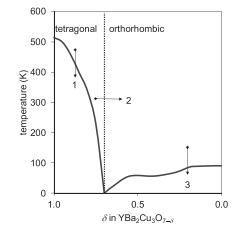
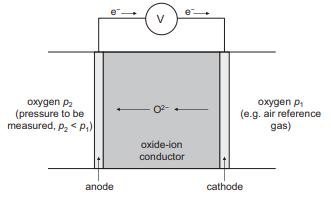
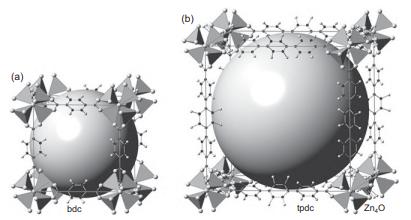
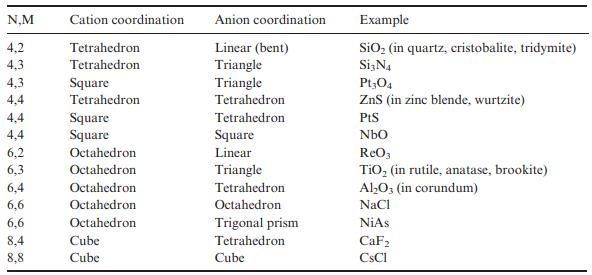
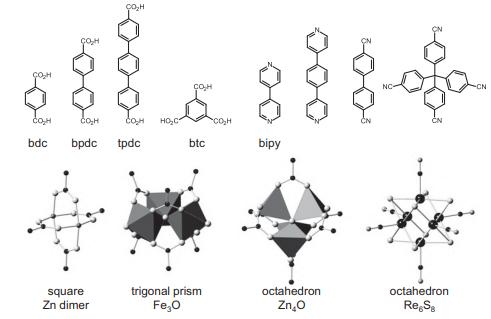
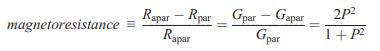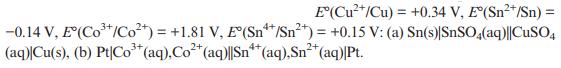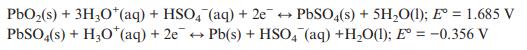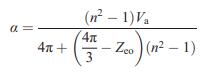Solid State Materials Chemistry 1st Edition Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt - Solutions
Discover the ultimate resource for "Solid State Materials Chemistry 1st Edition" by Patrick M. Woodward and others. Access online solutions with our comprehensive answers key and detailed solutions manual. Explore solved problems and test banks to enhance your understanding of the textbook. Our chapter solutions and step-by-step answers provide clarity on complex topics, ideal for both students and instructors. Benefit from the instructor manual to guide your teaching process. Enjoy the convenience of free download options for solutions PDFs, offering questions and answers at your fingertips. Unlock the potential of your studies with our expertly curated material.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()