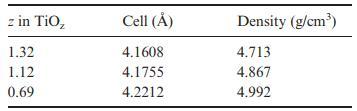
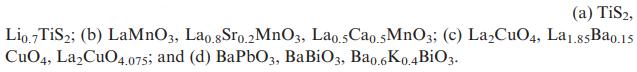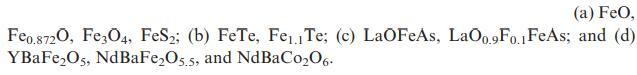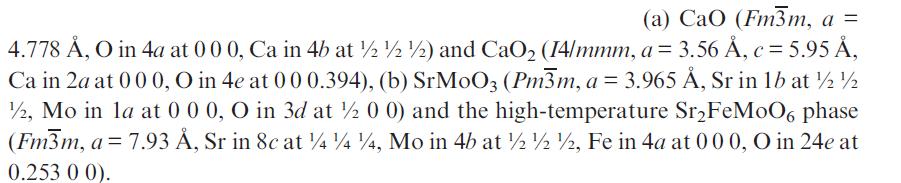![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


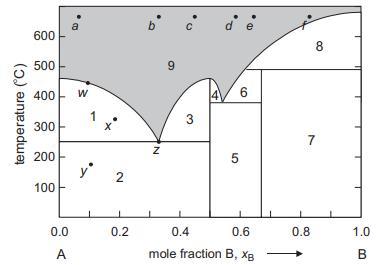
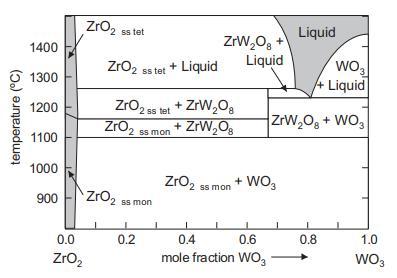


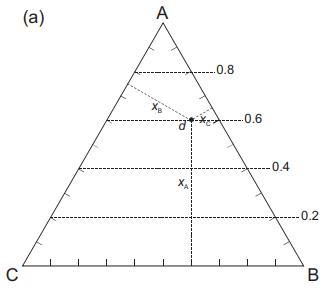
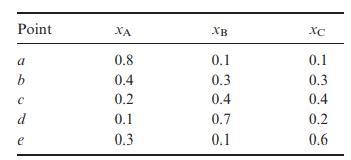
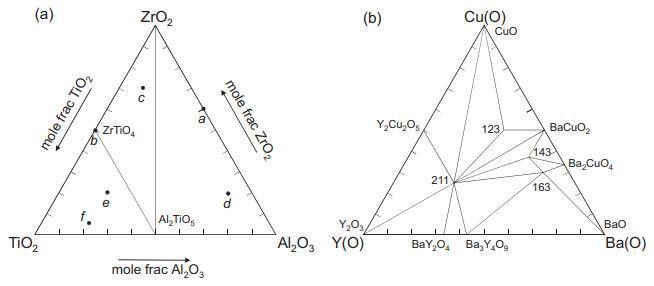
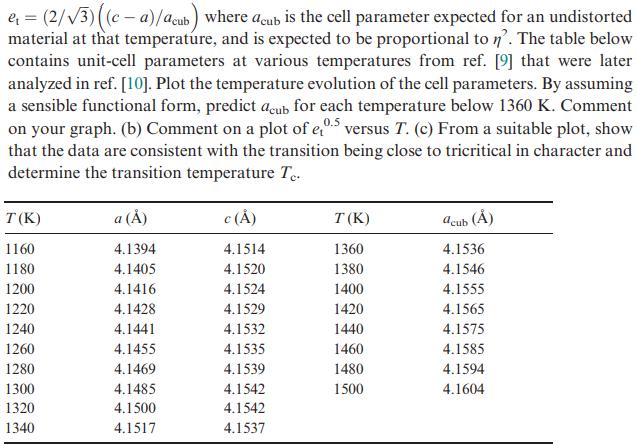
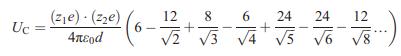
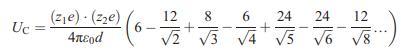


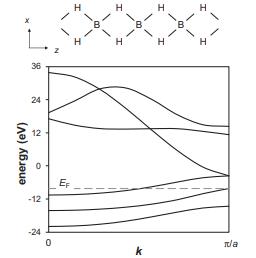
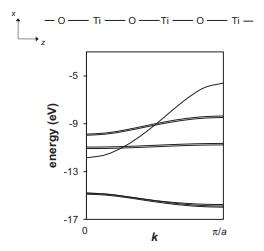
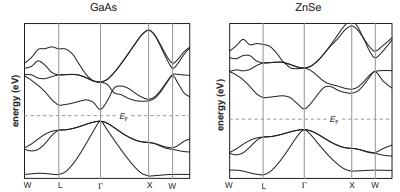
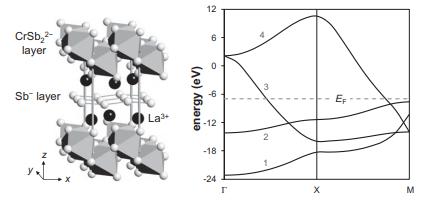
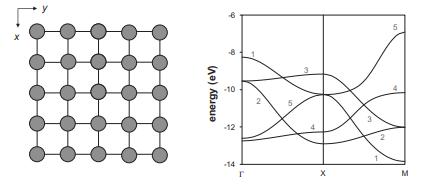
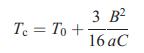
![VNI h ionic defects dominate reduction [v]= [VNI stoichiometric range oxidation h Ni electronic defects](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1705/7/4/1/36365ab8c331e9151705741359969.jpg)